Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagai, Takayuki; Okamoto, Yoshihiro; Yamagishi, Hirona*; Shibata, Daisuke*; Kojima, Kazuo*; Hasegawa, Takehiko*; Sato, Seiichi*; Fukaya, Akane*; Hatakeyama, Kiyoshi*
JAEA-Research 2023-004, 45 Pages, 2023/09
The local structure of glass-forming elements and waste elements in borosilicate glasses varies with its chemical composition. In this study, simulated waste glass samples were prepared and the chemical state regarding boron (B), silicon (Si) and waste elements of iron (Fe), cesium (Cs) were estimated by using XAFS measurement in soft X-ray region. To understand the chemical stability of simulated waste glasses, XANES spectra of B K-edge, Fe L , L
, L -edge, and Cs M
-edge, and Cs M , M
, M -edge were measured on the glass surface exposed to the leachate. As a result, it was found that the glass surface exposed to the leachate was changed and it was difficult to obtain a clear XANES spectrum. From the B K-edge XANES spectra on glass surfaces exposed to the leachate, an increase in three-coordination of B-O (BO
-edge were measured on the glass surface exposed to the leachate. As a result, it was found that the glass surface exposed to the leachate was changed and it was difficult to obtain a clear XANES spectrum. From the B K-edge XANES spectra on glass surfaces exposed to the leachate, an increase in three-coordination of B-O (BO ) and a decrease in four-coordination of B-O (BO
) and a decrease in four-coordination of B-O (BO ) were observed compared to the glass surfaces before immersion. The XANES spectra of Fe L
) were observed compared to the glass surfaces before immersion. The XANES spectra of Fe L , L
, L -edge, and Cs M
-edge, and Cs M , M
, M -edge show that as the exposure time in the leachate increases, the Cs present on the glass surface dissolves into the leachate. The XANES spectra of Si K-edge were measured on simulated waste glass surfaces before immersion, and it was confirmed that the change in XANES spectra given by Na
-edge show that as the exposure time in the leachate increases, the Cs present on the glass surface dissolves into the leachate. The XANES spectra of Si K-edge were measured on simulated waste glass surfaces before immersion, and it was confirmed that the change in XANES spectra given by Na O concentration had a larger effect than the waste component concentration.
O concentration had a larger effect than the waste component concentration.
Onuki, Toshihiko*; Ye, J.*; Kato, Tomoaki; Liu, J.; Takano, Masahide; Kozai, Naofumi; Utsunomiya, Satoshi*
Environmental Science; Processes & Impacts, 25(7), p.1204 - 1212, 2023/07
Times Cited Count:0 Percentile:0(Chemistry, Analytical)To elucidate chemical forms of Cs and I in microparticles produced via the Fukushima Daiichi Nuclear Power Plant accident and released into the atmosphere, we analyzed Cs and I in condensed vaporized particles (CVP) produced by melting experiments using nuclear fuel components containing CsI with concrete. CVPs consisted of many round particles containing Cs and I of diameters less than several tens of micrometers. Two kinds of particles were present: one containing large amounts of Cs and I, suggesting the presence of CsI, and the other containing small amounts of Cs and I with large Si contents. Most of CsI from both particles were dissolved in water. On the contrary, some fractions of Cs remained from the latter particles. These results suggest that Cs was incorporated in CVPs along with Si to form water low-soluble CVPs
Nagai, Takayuki
JAEA-Research 2022-014, 84 Pages, 2023/02
Most of the simulated waste glasses used for physical property evaluation are processed into a shape suitable for the measurement method from glass gob obtained by slowly cooling molten glass to room temperature. However, the actual vitrified waste glass material is obtained by cooling and being coagulated the glass drained from the bottom of glass melter into the canister. In this study, Raman spectroscopy was performed on the coagulated surface of molten simulated waste glass in the depth direction to evaluate the state of the Si-O bridging structure near the coagulated glass surface. The Raman spectra measured from the surface to the depth direction near the surface of the glasses produced by several melting and coagulation conditions of molten simulated waste glass cullet in the air atmosphere, and it was confirmed that there were changes in these spectra. On the other hand, the raw material glass cullet and the surface of the glass solidified in argon gas atmosphere showed little change in the spectrum in the depth direction, and the Si-O bridging structure near the glass surface was similar. It was also confirmed that the spectrum change in the depth direction measurement was small for the cut surface of the glass, and that the change in the spectrum for the broken glass fracture surface was also small. For glasses with a large change in Raman spectra in the depth direction near the coagulated surface, the molten glass was cooled from the molten state to room temperature in a muffle furnace with air atmosphere. That is, the magnitude of the spectral change with respect to the depth direction depends on the time from the molten state to coagulation. In order to confirm the reason why the number of bridging oxygen in the Si-O bridging structure is small on the coagulated surface of glass, the XANES spectra of Si-K edge and Ce-L3 edge were measured by XAFS on the coagulated surface and the cutting face. As a result, the Si-K edge peak on the coagulated surface is
Sasaki, Yuji; Nakase, Masahiko*; Kaneko, Masashi; Kobayashi, Toru; Takeshita, Kenji*; Matsumiya, Masahiko*
Analytical Sciences, 5 Pages, 2023/00
Times Cited Count:0 Percentile:0(Chemistry, Analytical)We conducted three field researches on Ru-extraction, XANES, and DFT-calculation. The order of the distribution ratio, D(Ru), from acid, HCl  H
H SO
SO
 HNO
HNO
 HClO
HClO , by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide
, by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide  MIDOA
MIDOA  IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
 Ln
Ln O
O (Ln = Gd, Er) solid solution
(Ln = Gd, Er) solid solutionPham, V. M.*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Akiyama, Daisuke*; Nagai, Takayuki; Okamoto, Yoshihiro
Journal of Nuclear Materials, 556, p.153189_1 - 153189_9, 2021/12
Times Cited Count:1 Percentile:16.35(Materials Science, Multidisciplinary)The crystal structure change was evaluated for (1-x)UO -xLnO
-xLnO (Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H
(Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H atmospheres. The effect of LnO
atmospheres. The effect of LnO doping on the crystal structure of UO
doping on the crystal structure of UO was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO
was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO doping into UO
doping into UO reduced the lattice parameter of UO
reduced the lattice parameter of UO -LnO
-LnO solid solutions up to 40mol% LnO
solid solutions up to 40mol% LnO . The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O
. The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O solid solutions, that is, the O/M ratios were close to 2.00. The U L
solid solutions, that is, the O/M ratios were close to 2.00. The U L -edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
-edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
Sugiura, Yuki; Tomura, Tsutomu*; Ishidera, Takamitsu; Doi, Reisuke; Francisco, P. C. M.; Shiwaku, Hideaki; Kobayashi, Toru; Matsumura, Daiju; Takahashi, Yoshio*; Tachi, Yukio
Journal of Radioanalytical and Nuclear Chemistry, 324(2), p.615 - 622, 2020/05
Times Cited Count:4 Percentile:45.45(Chemistry, Analytical)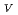 -edge XANES to determination of U oxidation state in zircon
-edge XANES to determination of U oxidation state in zirconTanaka, Kazuya; Takahashi, Yoshio*
Geochemical Journal, 53(5), p.329 - 331, 2019/00
Times Cited Count:0 Percentile:0.01(Geochemistry & Geophysics)We examined three natural zircon samples with different amounts of radiation doses using M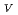 -edge and L
-edge and L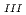 -edge U X-ray absorption near-edge structure (XANES). Analysis of XANES spectra at both M
-edge U X-ray absorption near-edge structure (XANES). Analysis of XANES spectra at both M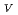 -edge and L
-edge and L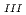 -edge suggested that the oxidation state of U in the zircon sample with the highest radiation dose is tetravalent. The XANES spectra of the two other samples with lower radiation doses suggested a mixture of U(IV) and U(VI), while the possibility of U(V) was not excluded. This is the first work on the application of M
-edge suggested that the oxidation state of U in the zircon sample with the highest radiation dose is tetravalent. The XANES spectra of the two other samples with lower radiation doses suggested a mixture of U(IV) and U(VI), while the possibility of U(V) was not excluded. This is the first work on the application of M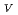 -edge U XANES to the oxidation state of U in natural zircon.
-edge U XANES to the oxidation state of U in natural zircon.
 -Edge X-ray absorption near edge structure spectra
-Edge X-ray absorption near edge structure spectraOta, Atsuyuki*; Tanaka, Kazuya; Tsuno, Hiroshi*
Journal of Physical Chemistry A, 122(41), p.8152 - 8161, 2018/10
Times Cited Count:1 Percentile:3.34(Chemistry, Physical)We investigated the application of L -edge XANES spectra to the local structural analysis of lanthanoids in aqueous solution, iron hydroxide, manganese dioxide, and calcium carbonate. For each lanthanoid, the full width at half maximum (FWHM) values of lanthanoid compounds roughly decreased with increasing coordination numbers. However, they did not strictly reflect the local coordination sphere of the lanthanoid complex, but were rather sensitive to their chemical forms. The relationship between the magnitude of the FWHM values was determined by the crystal field splitting or degeneracy of 5d orbitals. The systematic variation of FWHM can be explained by the ligand strength of the ligand molecules (-H
-edge XANES spectra to the local structural analysis of lanthanoids in aqueous solution, iron hydroxide, manganese dioxide, and calcium carbonate. For each lanthanoid, the full width at half maximum (FWHM) values of lanthanoid compounds roughly decreased with increasing coordination numbers. However, they did not strictly reflect the local coordination sphere of the lanthanoid complex, but were rather sensitive to their chemical forms. The relationship between the magnitude of the FWHM values was determined by the crystal field splitting or degeneracy of 5d orbitals. The systematic variation of FWHM can be explained by the ligand strength of the ligand molecules (-H O
O , -O
, -O , -OH
, -OH , -CO
, -CO
 , -Cl
, -Cl , and -O
, and -O ) that cause the crystal field splitting. Therefore, the FWHM values of L
) that cause the crystal field splitting. Therefore, the FWHM values of L -edge XANES of lanthanoid compounds may be more useful in speciation analysis rather than structural analysis such as EXAFS.
-edge XANES of lanthanoid compounds may be more useful in speciation analysis rather than structural analysis such as EXAFS.
Shimada, Hiroyuki*; Fukao, Taishi*; Minami, Hirotake*; Ukai, Masatoshi*; Fujii, Kentaro; Yokoya, Akinari; Fukuda, Yoshihiro*; Saito, Yuji
Journal of Chemical Physics, 141(5), p.055102_1 - 055102_8, 2014/08
Times Cited Count:16 Percentile:53.96(Chemistry, Physical)Konishi, Hiroyuki; Yamashita, Masato*; Uchida, Hitoshi*; Mizuki, Junichiro
Materials Transactions, 46(1), p.136 - 139, 2005/01
Times Cited Count:2 Percentile:25.81(Materials Science, Multidisciplinary)The rust layer formed on weathering steel possesses a strong protective ability against corrosives in an atmosphere. This ability is related to the structure of the rust layer. The difference in the protective ability of a rust layer in a Cl-rich environment between conventional weathering steel containing Cr and advanced weathering steel containing Ni is believed to be caused by the differences in local structural and chemical properties between alloying elements, Cr and Ni, in the rust layer. In order to examine the effect of these alloying elements on the structure of the rust layer formed on steel in a Cl-rich environment, we have performed Cr and Ni K-edge X-ray absorption near-edge structure (XANES) measurements for the rust layer of Fe-Cr and Fe-Ni binary alloys exposed to a Cl-rich atmosphere using synchrotron radiation. The results of the Cr K-edge XANES measurements for the rust layer of Fe-Cr binary alloys show that the atomic geometry around Cr depends on the concentration of Cr. Therefore, it is expected that the local structure around Cr in the rust layer is unstable. On the other hand, from the results of the Ni K-edge XANES measurements for the rust layer of Fe-Ni binary alloys, Ni is considered to be positioned at a specific site in the crystal structure of a constituent of the rust layer, such as akagan ite or magnetite. As a consequence, Ni negligibly interacts with Cl
ite or magnetite. As a consequence, Ni negligibly interacts with Cl ions in the rust layer.
ions in the rust layer.
Yaita, Tsuyoshi; Shiwaku, Hideaki; Suzuki, Shinichi; Okamoto, Yoshihiro; Shimada, Asako*; Assefa, Z.*; Haire, R. G.*
Physica Scripta, T115, p.302 - 305, 2005/00
The structural parameter and the electronic structure of the complex between cerium and N,N'-dimethyl-N,N'-diphenylpyridine-2,6-carboxyamide(DMDPhPDA) was investigated by XAFS, photo luminescence and excitation spectra. The DMDPhPDA is one of the promising ligands for separation of trivalent actinides from lanthanides. The Ce-K XAFS spectra were measured at BL11XU of SPring-8. The bond distances between the carbonyl oxygens and cerium, and between the pyridyl nitrogen and cerium are 253pm and 264pm, respectively. The bond angle of the plane consisting of the two carbonyl oxygens and nitrogen with cerium was about 180 degree. This complex was a yellow color unique for the DMDPhPDA-lanthanide complexes. The photo absorption peak for the complex was extremely broad, and the peak position was at a higher wavenumber as compared with those for the other lanthanide complexes. The peak in the highest wavenumber of this band may be attributed to the transition between f and d orbitals, which are greatly influenced by the ligand field of the DMDPhPDA. The XANES spectrum of the cerium complex clearly showed that cerium was trivalent.
Yokoya, Akinari; Takakura, Kaoru*; Watanabe, Ritsuko; Akamatsu, Ken*; Ito, Takashi*
Radiation Research, 162(4), p.469 - 473, 2004/10
Times Cited Count:3 Percentile:10.08(Biology)X-ray absorption spectra from single crystals of 5-Bromouracil were measured with the transmission mode in the energy range from 13.41 to 13.50 keV using the linearly polarized synchrotron radiation (SR). A characteristic resonance structure, consisting of four peaks, was recognized in the spectra in the Br K-edge region. The intensities of these peaks were strongly dependent on the crystal rotation about the normal of the crystal b-c plane, which was set perpendicular to the X-ray beam direction. (SR X-rays are polarized in the horizontal plane.) Molecular orbital calculations indicate that these resonance peaks are associated with the transitions from the 1s electron of Br to the Br-C molecular antibonding orbitals and to a shape resonance. The observed anisotropy of each photoabsorption peak might originate from the angular dependences of these molecular orbitals.
Baba, Yuji; Sekiguchi, Tetsuhiro; Shimoyama, Iwao; Nath, K. G.
Applied Surface Science, 234(1-4), p.246 - 250, 2004/07
Times Cited Count:10 Percentile:46.84(Chemistry, Physical)no abstracts in English
 -edges
-edgesBaba, Yuji
Low Temperature Physics, 29(3), p.228 - 242, 2003/03
Times Cited Count:54 Percentile:51.76(Physics, Applied)no abstracts in English
Mizumaki, Masaichiro*; Yoshii, Kenji; Kitazawa, Hideaki*; Tanida, Hajime*
Journal of Solid State Chemistry, 171(1-2), p.291 - 294, 2003/02
Times Cited Count:5 Percentile:17.59(Chemistry, Inorganic & Nuclear)no abstracts in English
Yokoya, Akinari; Akamatsu, Ken*; Fujii, Kentaro
Nuclear Instruments and Methods in Physics Research B, 99, p.366 - 369, 2003/01
no abstracts in English
Fujii, Kentaro; Akamatsu, Ken; Muramatsu, Yasuji; Yokoya, Akinari
Nuclear Instruments and Methods in Physics Research B, 199, p.249 - 254, 2003/01
Times Cited Count:40 Percentile:91.19(Instruments & Instrumentation)no abstracts in English
Okamoto, Yoshihiro; Akabori, Mitsuo; Ito, Akinori; Ogawa, Toru
Journal of Nuclear Science and Technology, 39(Suppl.3), p.638 - 641, 2002/11
We report local structural features of molten UCl with LiCl-KCl eutectic probed by the U L
with LiCl-KCl eutectic probed by the U L -edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U
-edge XAFS(X-ray absorption fine structure). The XAFS measurements were performed in a transmission mode at the BL27B station of the Photon Factory(High Energy Accelerator Organization, Tsukuba, JAPAN). Sample prepared by chlorination of uranium hydride and then reduction with zinc powder was sealed in a quartz cell under reduced pressure. The nearest U -Cl
-Cl distance and the coordination number of Cl
distance and the coordination number of Cl around U
around U ion were obtained by a curve fitting of the 1st shell XAFS function k
ion were obtained by a curve fitting of the 1st shell XAFS function k
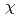 (k). The pair potential in the U
(k). The pair potential in the U -Cl
-Cl correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
correlation was evaluated from XAFS simulation by combinational use of the MD and the FEFF8. In addition, valence state of uranium in the melt was evaluated by XANES(X-ray absorption near edge structure) spectra.
Baba, Yuji; Sekiguchi, Tetsuhiro; Shimoyama, Iwao
Nuclear Instruments and Methods in Physics Research B, 194(1), p.41 - 46, 2002/07
Times Cited Count:4 Percentile:29.4(Instruments & Instrumentation)no abstracts in English
Akiyama, Kazuhiko; Sueki, Keisuke*; Tsukada, Kazuaki; Yaita, Tsuyoshi; Miyake, Yoko*; Haba, Hiromitsu*; Asai, Masato; Kodama, Takeshi*; Kikuchi, Koichi*; Otsuki, Tsutomu*; et al.
Journal of Nuclear and Radiochemical Sciences, 3(1), p.151 - 154, 2002/06
The oxidation state of actinide elements encapsulated in fullerenes is studied. HPLC elution behavior of actinide-fullerenes is classified into two groups; the elution behavior of the first group, encapsulating U, Np, and Am, is similar to that of the light lanthanide-fullerenes, such as La, Ce, Pr, and Nd, while the behavior of the second group, encapsulating Th and Pa, is quite different from that of any lanthanide-fullerenes. The chemical species in the main HPLC elution peak of each group were identified as M@C82 and M@C84 (M = metal atom) from the mass of the U and Th fullerenes, respectively. The oxidation states of the U and Th atoms in the fullerenes were deduced to be 3+ and 4+, respectively, from the UV/vis/NIR absorption and XANES spectroscopy.